Background: Prolonged hospitalization following intensive (re)induction chemotherapy until resolution of cytopenias is the current standard of care for adults with acute myeloid leukemia (AML), but this approach is costly, resource intense, contributory to hospital bed shortages, and negatively impacts patients' quality of life (QOL). Early hospital discharge (EHD) immediately following completion of chemotherapy has been proposed as an alternative and has proven safe at select institutions. Here, we conducted a National Comprehensive Cancer Center (NCCN) survey, through its Best Practices Committee (BPC), to examine discharge practices for adults with AML at other major cancer centers to better understand interest in, and barriers to, adopting an EHD approach more broadly.
Methods: NCCN is a non-profit alliance of 33 cancer centers in the U.S. The survey was developed by 2 physicians at one center and piloted at 2 other centers. The NCCN BPC, which is composed of senior physician, nursing, and administrative leaders from NCCN Member Institutions, distributed the survey in early 2023 via a web-based survey tool (SurveyMonkey, San Mateo, CA) to a representative at each center, requesting completion by a content area expert. No incentives were offered for completion. Data were analyzed using descriptive statistics.
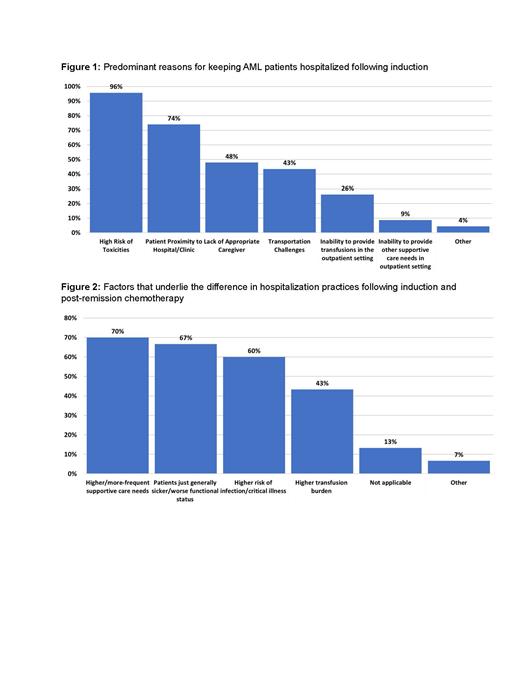
Results: 30/33 (91%) centers completed the survey, 60% of which treat >100 AML patients annually and 45% treat over half of their patients with intensive chemotherapy (7+3 or regimens with a high-dose cytarabine backbone). All centers perform allogeneic hematopoietic cell transplantations (HCT). There was no difference in the proportion of intensively treated patients across centers by patient volume. Following (re)induction therapy, 80% of institutions keep patients hospitalized until blood count recovery, whereas 20% attempt to discharge patients early if medically stable and logistics permit. Concerns regarding patient safety, housing, and caregiver availability were the main reasons for keeping patients hospitalized ( Figure 1). In contrast, following post-remission (“consolidation”) therapy, 87% of centers discharge patients early. Concerns regarding increased care needs, worse functional status, and higher complications risks following induction relative to post-remission therapy accounted for the main reasons for this difference ( Figure 2). 80% of centers were interested in exploring EHD after (re)induction but noted barriers including affordable local housing (71%), transportation (50%), high toxicity/infection rate (50%), high transfusion burden (50%) and limited bed availability when re-hospitalization is necessary (50%). Among the 6 centers that practice EHD, all have infusion centers with full weekday hours, 5 have full weekend/holiday hours, 3 more have evening hours, and 1 has reduced holiday/weekend hours. All can provide blood product support within 4 hours from ordering and administer IV antimicrobials within 1 hour from ordering in outpatient clinic. In 4 centers, evaluation of a patient with presumed neutropenia contacting the clinic with fever during regular hours can occur in the outpatient clinic, with subsequent admission if necessary; at one center the patient would be referred to the ED and at one center the patient would be directly admitted to an inpatient ward. If a patient needs admission, a bed would be available for direct admission to the inpatient ward in 4/6 of the centers >75% of the time, at 1 center 26-50% of the time, and at 1 center <25% of the time. EHD was not practiced at any centers in cities with population of >1 million, citing local housing availability as a major EHD barrier. There was no correlation between the ability to practice EHD and annual AML patient volume, treatment intensity patterns, or hospitalization practices following HCT.
Conclusions: Hospitalization and care patterns following AML therapy are highly variable across major U.S. cancer institutions. While only 20% of major cancer centers surveyed practice EHD following intensive (re)induction chemotherapy, 87% do so following post-remission therapy. There is interest in exploring this approach despite medical and logistical barriers. Given potential advantages of EHD for both patients and healthcare systems, strategies to address these barriers should be explored to reduce healthcare resource utilization and improve patients' QOL.
Disclosures
Halpern:Abbie, Notable Labs, Agios: Consultancy; Imago Bioscience, Bayer, Gilead, Jazz, Incyte, Karyopharm Therapeutics, Disc Medicine: Research Funding. Stewart:Glycomimetics: Other: Spouse, research funding; Pfizer: Other: Spouse, research funding; GPCR: Other: Spouse, research funding; Notable labs: Other: Spouse, research funding; Accordand Health Services/CVS Caremark: Other: Spouse, Medical advisor. Walter:ImmunoGen, Jura: Consultancy, Research Funding; Abbvie, Adicet, Amphivena, BerGenBio, Bristol Myers Squibb, GlaxoSmithKline, Orum: Consultancy; Amgen, Aptevo, Celgene, Janssen, Jazz, MacroGenics, Pfizer: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal